 Image credit: Carlos Reding
Image credit: Carlos Reding
 Image credit: Carlos Reding
Image credit: Carlos Reding
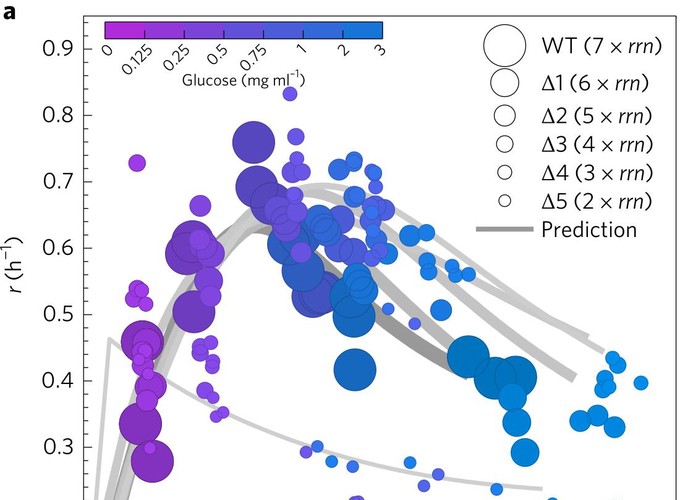
Evolutionary trajectories are constrained by trade-offs when mutations that benefit one life history trait incur fitness costs in other traits. As resistance to tetracycline antibiotics by increased efflux can be associated with an increase in length of the Escherichia coli chromosome of 10% or more, we sought costs of resistance associated with doxycycline. However, it was difficult to identify any because the growth rate (r), carrying capacity (K) and drug efflux rate of E. coli increased during evolutionary experiments where the species was exposed to doxycycline. Moreover, these improvements remained following drug withdrawal. We sought mechanisms for this seemingly unconstrained adaptation, particularly as these traits ought to trade-off according to rK selection theory. Using prokaryote and eukaryote microorganisms, including clinical pathogens, we show that r and K can trade-off, but need not, because of ‘rK trade-ups’. r and K trade-off only in sufficiently carbon-rich environments where growth is inefficient. We then used E. coli ribosomal RNA (rRNA) knockouts to determine specific mutations, namely changes in rRNA operon (rrn) copy number, than can simultaneously maximize r and K. The optimal genome has fewer operons, and therefore fewer functional ribosomes, than the ancestral strain. It is, therefore, unsurprising for r-adaptation in the presence of a ribosome-inhibiting antibiotic, doxycycline, to also increase population size. We found two costs for this improvement: an elongated lag phase and the loss of stress protection genes.